“Exploiting scarcity is not ethical,” WHO wrote in its statement today.
dangerous test
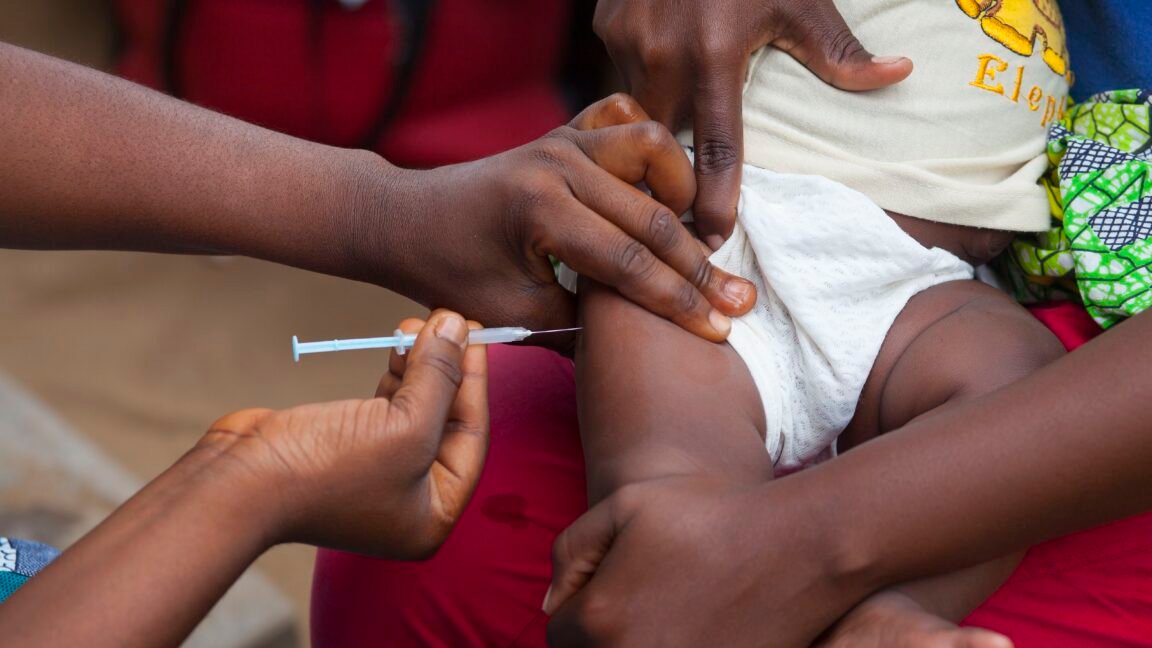
The UN health agency highlighted that the hepatitis B vaccine birth dose is “an effective, and essential public health intervention” that has been used “for more than three decades, with more than 115 countries including it in their national programs.”
“It prevents life-threatening liver disease by preventing mother-to-child transmission at birth,” WHO wrote, noting that more than 12 percent of adults in Guinea-Bissau have chronic hepatitis B.
In the subtitle “Why stopping the vaccine is unethical”, WHO outlines all the reasons why the trial is dangerous.
“From what has been described publicly, [trial] “It appears that the protocol does not ensure even a minimal level of harm reduction and benefit to study participants (for example, screening pregnant women and vaccinating newborns exposed to hepatitis B),” WHO wrote.
WHO argues that as a proven life-saving vaccine, withholding it from some study participants could cause serious and potentially irreversible harm to newborns, including chronic infections, cirrhosis and liver cancer. There is no scientific justification for stopping any proven intervention, and no credible evidence of the safety concerns that Benn and his colleagues claim to have found in their trial. WHO also noted that publicly available information about the trial indicated that it would be a single-blind, no-treatment-controlled design, which “raises a significant potential for substantial risk of bias, limiting the interpretation of the study results and their policy relevance.”
For now, the case seems to be stalled. Nature News reported that at a January 22 press conference, health officials in Guinea-Bissau said a technical and ethical review was pending. “There has not been enough coordination to make a final decision regarding the study,” said Quinhine Nantotte, Guinea-Bissau’s public health minister. “Facing this situation, we decided to suspend it.”
Previously, the Africa Centers for Disease Control and Prevention had suggested that the trial not proceed. However, the US Department of Health and Human Services issued a statement saying it was “proceeding as planned.”
<a href