It began, as many idea exercises do, with a discussion in the kitchen. My wife and I were debating whether a cooked egg was still safe to eat after spending the night on the kitchen counter. I’m definitely not one of those people who throws away food at even the slightest hint of an issue. I told her with full confidence that the egg would be fine. After all, egg shells are made of calcium carbonate, solid, sealed, and, in my opinion, impervious to any contaminants.
This proved to be one of those “smart idiot” moments. By the end of this journey, I will know how wrong I was, and how remarkable a life in engineering safety is.

My background is in sterile large molecule manufacturing within biotech, the kind of work where even a single microscopic breach within thousands of gaskets and kilometers of stainless steel can trigger an investigation lasting months or years. I’ve spent most of my career designing systems to keep pollutants out and detect them once they find a way in.
Yet after this curiosity-driven journey to see the effort, precision and technology we put into security, I came to the conclusion that nothing I have seen in biotech can compare to what nature spontaneously achieves inside an egg. To keep things simple, I focused on one scenario: an unfertilized, whole hen’s egg.
As I went down the rabbit hole of EggSecurity, I discovered that Egg is a surprisingly well-designed fortress. Before getting into the details, it’s worth noting just how packed with nutrients the egg actually is. It is so abundant in life-sustaining ingredients that even today, many biopharmaceutical products are made using eggs. For a bacterium to reach the egg yolk would be like winning the lottery. After all, the purpose of the yolk is to nurture life.
My first question was simple: Are egg shells porous? This shows that they are not only porous, but also contain thousands of microscopic pores. The average pore size is in the single-digit μm range. This may seem small, but it is still large enough for a tiny bacterium to pass through. For reference, sterile filtration uses filters with a pore size of 0.2 microns. I also know some aseptic subject matter experts who would argue for 0.1 μm to ensure that no microbials can penetrate. The benchmark for this comes from the smallest known bacterium used to challenge filter integrity, brevundimonas diminutaWith a rod-like shape and a diameter of 0.5 to 0.8 micrometres. This organism is introduced in high concentrations (10)7 colony forming units/cm2) to challenge the system.
My next question was what does it mean to pass through these holes? The answer was very clear. How could I have missed this? Biology 101: Good exchange of oxygen and carbon dioxide is essential for fetal development. Therefore the egg must allow gas molecules to pass through while keeping everything else out. How does the Ordinary Egg achieve this?
The first protective barrier is the antimicrobial cuticle, a thin outer coating that acts like a natural sealant. Whether it remains intact or partially washed away, it is the egg’s first line of defense. In countries where eggs are washed, this layer does not disappear immediately, so for a certain period of time it will still provide some antimicrobial protection.
Below this there is an eggshell with thousands of microscopic holes. These tunnels are not straight shafts but winding labyrinths, which are long and twisted. The environment inside is dry, making it difficult for bacteria to move. The dry environment of the eggshell also does not seem rich in nutrients to me, meaning it would be difficult for contaminants to move through the tunnel, which is another means of transport for microorganisms. Microorganisms thrive in warm, moist, and nutrient-rich environments. In a dry maze without air flow or nutrients, they must perform the microbial equivalent of cave exploration by remaining motionless and hungry in search of a hidden treasure.
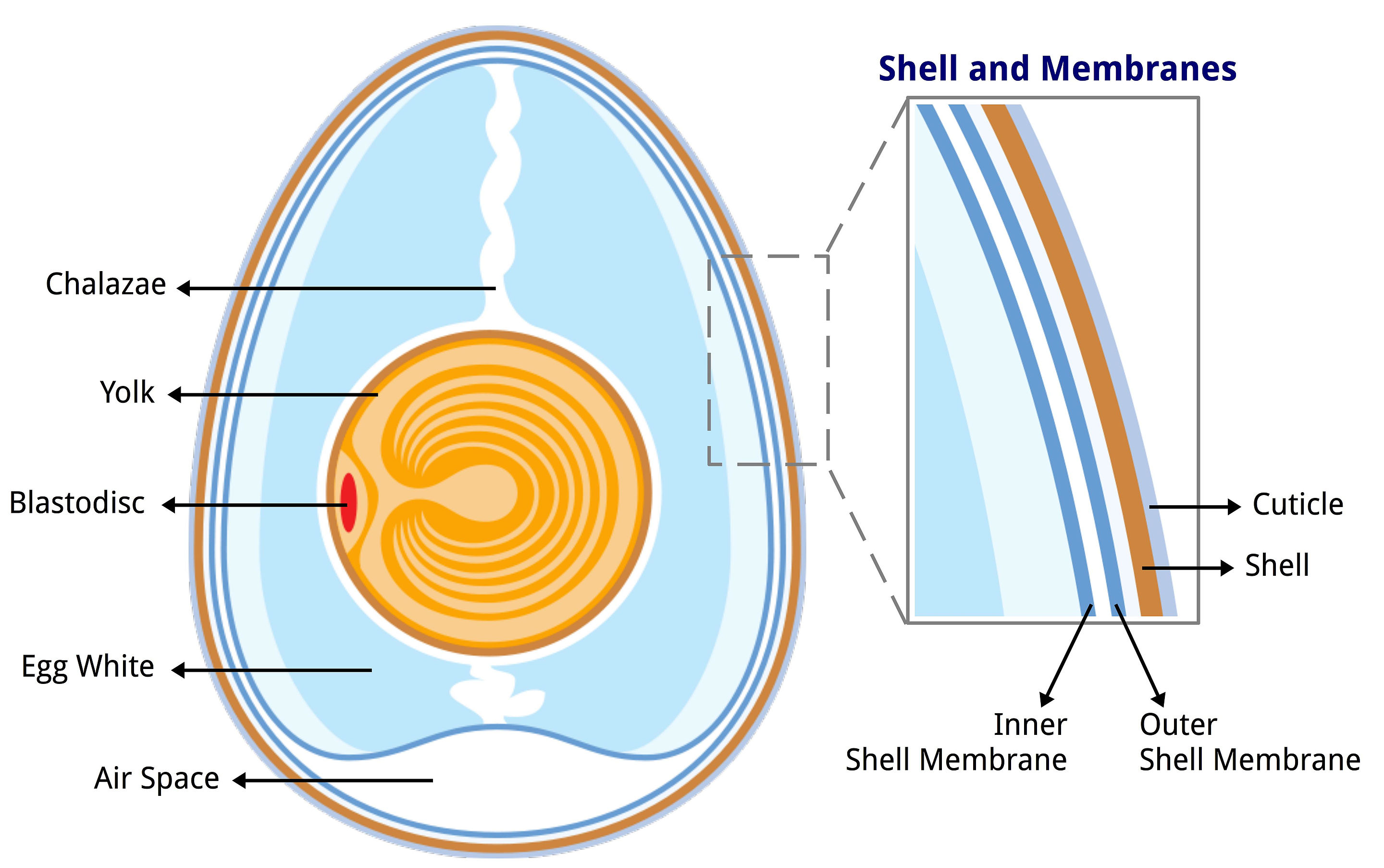
Each labeled layer forms part of the egg’s multi-level independent protection system.
Beyond the shell is the outer membrane, which is another fibrous layer with antimicrobial properties. The outer and inner membranes are separated by an air gap, which provides another layer of protection. This gap, like a ravine without a bridge, presents a challenge to bacteria. I think the outer membrane contains more nutrients than the egg shell, which means a bacterial colony can develop a bridge by consuming and multiplying within it. The inner membrane that follows presents another barrier, once again equipped with antimicrobial protection.
And then, the egg whites. Now it’s party time, right? The polluter has made it to the promised land. Not at all right now. Let’s assume it took a while for the bacteria to get here. Over time, the pH of egg whites increases. If it takes a week for the bacteria to break down the first protection, the pH may be around 9, which is definitely not ideal for bacterial growth. In addition, egg whites contain many antimicrobial components, which can be described as Batman’s utility belt.
Should a bacterium somehow survive, it must still breach one final layer, the vitelline membrane, and overcome yet another set of antimicrobial components, before moving straight down and reaching its final destination. The yolk, with its rich golden color, is the prize. A nutrient-rich storehouse optimized for life, packed with proteins and fats. This is heaven for any microbe.
So, back to the question: Was a cooked egg safe after spending a night on the counter? At first glance it seemed so. After all, the fort seemed impregnable. But the cooking had a fatal flaw. Heat denatures most of those antimicrobial proteins, leaving only misconfigured skeletons. As the egg cools, the contraction of the contents can create a negative pressure and draw in outside air to equalize, and with it, potential contaminants.
I thought to myself, as I have done many times at work, never underestimate the ingenuity of a contaminant. Then I had an aha moment and realized I didn’t even need to go down the rabbit hole, because this particular egg shell had a hole drilled in it for the egg cooker, compromising most of the protection layers.
Still, I really enjoyed this curiosity-driven trip. I realized that the egg might be the most extraordinary natural defense system ever created. Each layer has its own weaknesses: the cuticle can be washed away, the shell can absorb moisture under high humidity, the membrane can become old, and the albumin is only slowed down rather than destroyed, but the brilliance lies in their independence. If one layer fails, the other layers remain functional.
On the contrary it reminds me of engineered systems that boast layers of security but collapse when one component fails. We see this in the news about the recent Louvre robberies where some weak point led to the historic breach. On the other hand, the egg is not dependent on a chain where a broken link brings about a fall. It is a network of independent chains, each designed to operate autonomously. What’s even more remarkable when you think about it is that all of this is done without any constant energy input. Once the egg is laid, that’s it.
Ultimately, the lesson I learned from this is that the environments we design should be like egg yolks, rich, supportive, and full of growth potential at its core. Cores require layers of protection, invisible but flexible, that protect them from damage. To bad actors, the system should feel like an egg-layered fortress: complex, unyielding, hostile, and nearly impossible to penetrate.
Of course, this is easier said than done. But if nature can build such a perfect fortress, surely we can strive to do the same for the world we create for people and against bad actors.
I would be curious to hear your thoughts or if I have made any misconceptions. I’m especially interested to hear from egg experts and whether you’re as intrigued by the humble egg as I am!
